U.S. FDA Issues Extension on Nutrition Fact Label Requirements
On May 27, 2016, the Food and Drug Administration (FDA) submitted the Nutrition Facts Label Rule and the Serving Size Final Rule to update nutrition requirements found on food labels. This rule was first set with a compliance date set for July 26, 2018 at the earliest. These final rules demand food manufacturers help the public make better and more informed choices with their food. On July 3, 2018, the FDA extended the compliance dates by 18 months. Although 29,000 products have already implemented these changes, other manufacturers’ updates are still in progress. Let’s look at how these changes will affect how we read food labels.
“Working to improve the nutrition and diet of Americans can be a transformative effort that helps reduce the burden of many chronic diseases, ranging from diabetes to cancer to heart disease. It’s just as important for consumers to be able to effectively use the updated food labels, and we’re launching a major educational campaign for consumers to help them better understand the new nutrition information that they’ll be seeing in the marketplace.”
– Scott Gottlieb, FDA Commissioner, M.D.
With some food label fact requirements up to 20 years old now, the FDA has created the Nutrition Facts Label Rule and Serving Size Rule to provide the public with more current information. This information is a result of scientific findings, nutrition and public health research, dietary recommendations, and public input. Among the groups taken into account when considering this change include the World Health Organization, Institute of Medicine, the American Academy of Pediatrics, and the American Heart Association.
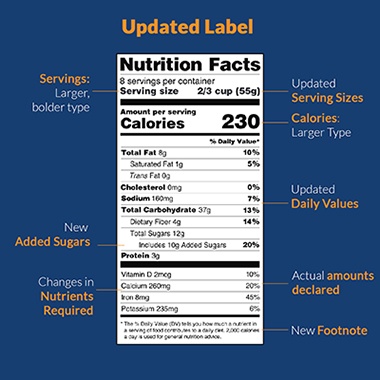
Nutrition Facts Label Final Rule
The Nutrition Facts Label Final Rules appears to offer the most noticeable and impactful changes between the two rules being discussed. The nutrition facts label acts as a comprehensive resource for buyers to learn about the nutritional content of the food they eat. Consequently, the FDA is trying to make it easier to read and understand these labels to allow more educated food choices.
The Nutrition Facts Label Final Rule revisions involve:
- Removing “Calories from Fat”
- Requiring the gram amount of “Added Sugars” per serving
- Amending “Sugars” to “Total Sugars”
- Updating lists of vitamins and minerals significant to public health
- Reformatting the label to make “Calories” more prominent
Serving Size Final Rule
In 1993, the FDA published what the public knows as serving size requirements on food labels. However, the amount of how much people eat and drink has changed in 25 years. Therefore, the Serving Size Rule will amend recommended serving sizes based on what consumers are actually eating instead of what they should be eating.
For products offered in serving sizes larger than single servings, like a 24-ounce bottle of soda, the food manufacturer will be required to provide “dual column” labels to illustrate the amount of nutrients and calories both “per serving” and “per package.”
“Package size affects what people eat. So for packages that are between one and two servings, such as a 20-ounce soda or a 15-ounce can of soup, the calories and other nutrients will be required to be labeled as one serving because people typically consume it in one sitting.”
– U.S. Food & Drug Administration (FDA)
Public reaction to proposed ruling
Given the national impact of the proposed rules, the FDA allowed a 30-day comment period to provide an opportunity for those in support and in opposition to the rules to share feedback. In those 30 days, the FDA received over 50,000 comments from consumer groups, food manufacturers, individual consumers, health professionals, academia, and a variety of others. To validate and support this feedback, the FDA has taken and will continue to take measures to ensure an accurate and efficient implementation.
How the FDA plans to address concerns
The first of these measures include the extension of the compliance dates. The compliance date for food manufacturers with $10 million or more in annual revenue is updated to January 1, 2020. For those with less than $10 million in annual revenue, the compliance date has shifted to January 1, 2021.
Secondly, the FDA will include educational materials to assist in helping consumers better understand nutrition facts label requirements. A task force will also be enlisted in combination with these materials to help develop, inform, and examine education efforts that target specific groups like low-literacy consumers, and populations with a higher risk of nutrition-related diseases.
UNE Online: M.S. Applied Nutrition
The U.S. Food and Drug Administration’s efforts to improve population health and use nutrition science to better inform consumers illustrates the increasing demand for experts in the field. UNE Online’s Master of Science in Applied Nutrition program provides a comprehensive education that can help support this transformation. Plus, the focus area model at UNE Online offers each student the benefit of shaping their degree around personal career goals. Where do you want to take your nutrition career next?
Click for more information on the Applied Nutrition program!
Tags: Applied Nutrition | Master of Science in Applied Nutrition